Learning Resources
L1: Naming and Writing Formulas for Hydrates
Lesson:
Ionic Hydrates
An ionic hydrate is a compound that has water associated with it. Water is part of its crystalline structure.
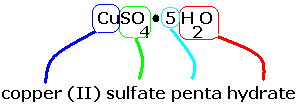
Bluestone (CuSO4 · 5 H2O) contains five water molecules per copper(II) ion and sulfate ion in the crystal.
Its molecular formula is CuSO4 · 5 H2O. Its IUPAC name is copper(II) sulfate pentahydrate.
The term anhydrous means without water, thus the name for anhydrous compound is simply copper(II) sulfate, and the molecular formula is CuSO4.
A hydrate like rock salt, sodium chloride monohydrate (NaCl·1H2O), can be formed when a salt water lake dries up leaving behind solid salt. However, not all of the water evaporates. Some water molecules became part of the salt crystals giving rise to hydrated crystals.
Writing Chemical Formulas for Ionic Hydrates:
The name of an ionic hydrate can be distinguished from the names of other ionic compounds by the presence of the term hydrate. For example, the IUPAC name for bluestone is copper(II) sulfate pentahydrate.
In order to convert IUPAC names for ionic hydrates into chemical formulas, you will need to know the prefix system learned earlier. Here is the list of these prefixes and the numbers associated with them just in case you have forgotten them.
| mono | = 1 |
| di | = 2 |
| tri | = 3 |
| tetra | = 4 |
| penta | = 5 |
| hexa | = 6 |
| hepta | = 7 |
| octa | = 8 |
| nona | = 9 |
| deca | = 10 |
Sample 1:
Write a chemical formula for each hydrate.
- sodium thiosulfate pentahydrate
- copper(II) sulfate pentahydrate
Answer
sodium thiosulfate pentahydrate
Na2S2O3 · 5 H2O. Notice that a dot separates water from the rest of the formula. |
cobalt (II) chloride dihydrate
CuCl2 · 2 H2O Again, notice that a dot separates water from the rest of the formula. |
Exercise 1:
- zinc sulphate heptahydrate
- potassium sulphate decahydrate
- Cadmium (II) nitrate tetrahydrate
Answers:
- ZnS04 · 7H20
- K2S04 · 10H20
- Cd(NO3)2 · 10H20
Writing Names for Ionic Hydrates:
Converting a chemical formula for an ionic hydrate into a name is a reversal of the steps you do to write the formula.
Sample Exercise:
Write the IUPAC names for these hydrates:
- Ni3(PO4)2·8H2O
- Fe(OH)3·3H2O
Answers:
Ni3(PO4)2·8H2O
Break the chemical formula into three parts.
· 8 H2O is octahydrate.
nickel(II) phosphate octahydrate. |
Fe(OH)3·3 H2O
Break the chemical formula into three parts.
OH- is the hydroxide ion.
the iron ion is iron(III).
·3H2O is trihydrate.
iron(III) hydroxide trihydrate. |
Again, note that it is a good idea to begin by locating the anion charge and name in case the cation is multivalent.
Exercise 2:
Give the formula for each of the following:
| 1. AlCl3 · 6 H2O |
| 2. MgSO4 · 7 H2O |
| 3. CuSO4 · 5H2O |
Answers:
- aluminum chloride hexahydrate
- magnesium sulphate heptahydrate
- copper(II) sulphate pentahydrate * (II) since copper has two possible charges !
Activity
Textbook Readings
Nelson Chemistry
- pages: 36
Textbook Items
Nelson Chemistry
- page 37: #1
- page 42: # 5
- page 45: #34,35, 54
- page 202: #52