Learning Resources
Lesson
Electrons and Charge
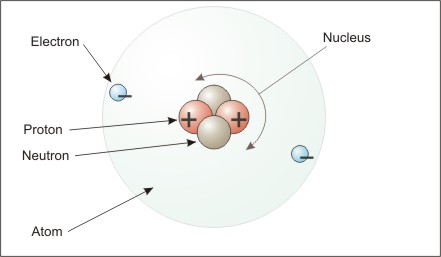
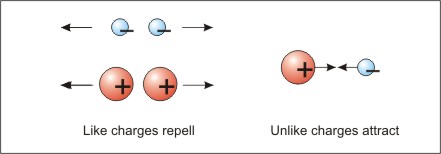
Electron movement is the essence of electricity. You may recall from earlier science courses, that all matter is composed of atoms, and that atoms are composed of a nucleus with protons and neutrons. Electrons are much smaller particles that orbit around the nucleus. Protons and electrons have a property called charge. Protons have a positive charge and electrons have a negative charge. Like charges repel each other, and unlike charges attract each other. Most of the time, the charges in an atom cancel each other out.
Figure Parts of an Atom
Figure Law of Electric Charges—Like Repels, Unlike Attracts
- Electron Charges. Electron charges are measured in units called coulombs. A coulomb is equal to the charge of 6,250,000,000,000,000,000 electrons (6.25 x 1018). This force was determined by Charles-Augustin Coulomb. Coulomb's Law essentially says that the force of attraction or repulsion between charged objects gets stronger if the objects get closer together, and if the amount of charge increases. As you will see in a later lesson, this is an important unit of measure and is the basis for other units of electrical measure.
Electricity
Electrons are always in motion. Because their motions are 'random' there is no net effect from the negative charges on them. When atmospheric conditions are right, a charge can accumulate, for example when you scuff over a carpet. The built-up electrons (negative charge) causes a spark and a snapping sound when it jumps from your hand to a door knob.
Electricity occurs when electrons flow in a more orderly manner from one location to another. Electron movement is no longer random so its effects are measurable and can be used to do useful things such as operate motors, lights, and radios.
Conductors, Insulators, and a Bit of Both
Some materials are made of atoms that have electrons held very tightly in orbit. For these materials, it is very difficult to get electrons to move from one atom to another. Such materials are called insulators. Other materials have electrons that can move very easily from one atom to another. These materials are called conductors. Both types of materials are important to the use of electricity. Conductors such as copper, aluminium, and gold allow you to have electrons flow easily, thereby allowing you to have electrical current where you want it. Insulators such as plastic, rubber, and ceramic allow you to prevent electrical current from going where you do not want it.
There is a special type of material called semiconductors that can be a good conductor under some circumstances, and a good insulator under others. They can, for example, allow current to flow in one direction and not the other or turn on or off when special conditions are met. Another type of materials called resistors will always conduct electricity, but will partially resist the flow of electrons.
Electrical Circuit
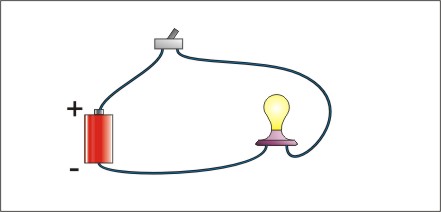
For electricity to flow, there must be a path that is a complete loop. The electrons have to get back to where they started, otherwise there is no electron flow, and no electricity. An electrical circuit has an energy source to create the electron flow, a conductor to provide a path for the electrical current, and a load (something that the electricity operates such as a light). Most circuits also have a control device such as a switch. The figure illustrates a simple circuit with a dry cell, a switch, a light bulb, and wires connecting it all together.
Figure Basic Electrical Circuit
Activity
Assigned activities
Research information on basic electricity concepts. Good starting places include
Test Yourself
There is no self test for this lesson.