| Intermediate Technology Education | Energy and Power Technology | Pre-Design | Topic 3 | Activity 8 |
Electric Potential Energy
- Define electrical potential difference, and state the unit of measure
- Define electrical current and state the unit of measure
Electric Energy
This activity covers the basic concepts related to electric potential energy in a non-technical way. For an excellent presentation of fundamental electrical principles, see the Physics Classroom Tutorial.
Electrical Potential Difference
Topics covered
- The structure of atoms, and the mobility of electrons
- Electric charge and the coulomb as a quantity of charge
- Potential difference
- Electromotive force (EMF)
- The unit of electromotive force (EMF)—the volt
Electricity Starts with Atoms
Atoms have, as part of their structure, the particles protons, neutrons, and electrons. Neutrons have no charge. Electrons are negatively charged and protons are positively charged. The figure below illustrates the principle.
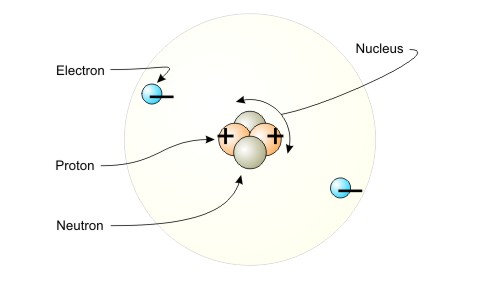
Figure Illustration of Atom
Negatively charged electrons and positively charged protons are the basis for electric charge.
Atoms generally have equal numbers of electrons and protons, so the positive and negative charges balance. Some atoms have electrons that can move from one atom to another. It is this mobility that is fundamental to electricity. Protons are part of the nucleus and cannot move from one atom to another.
Electric Charge And Quantity of Charge—the Coulomb
Electric charge is the basic building block of electricity. Electric charge occurs when there is an imbalance between the numbers of negative and positive charges in/on and object. Electric charges have these properties
- Like charges repel each other (negative repels negative, positive repels positive)
- Unlike charges attract each other (negative attracts positive and vice versa)
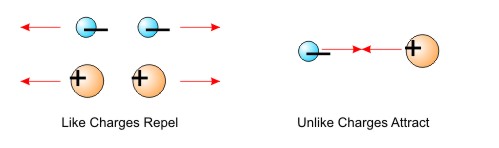
Figure The Properties of Charges are called the Law of Charges
Each electron has 1 unit of negative charge, and each proton has 1 unit of positive charge. A coulomb of electric charge is defined as 6.24 X 1018, or the total charge of 6.24 X 1018 electrons. The coulomb, named after Charles Augustin Coulomb (1736-1806), is a fundamental quantity on which all other measures of electricity are based.
Potential Difference
Potential difference refers to the difference in charge potential that occurs when one area has an excess of negative charge (too many electrons) and another has an excess of positive charge (shortage of electrons).
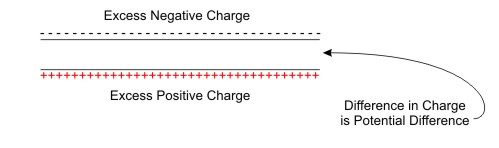
Figure Potential Difference occurs when there is an Imbalance in Charge between two Locations
Static electricity is an example of electric charge. Static charge can occur when certain materials rub together. You may have experienced static charge when fabrics stick together or to your skin, or gotten a shock when you touched a doorknob and the static charge on your skin jumped the short gap from your finger to the knob.
Electromotive Force (EMF)
The electric potential difference is called electromotive force, abbreviated to EMF. In the automotive battery shown below, the EMF is the total charge between the two terminals.
The Unit of Measure for EMF—The Volt
EMF has a unit of measure called the volt (named after Alessandro Volta, 1745-1827). By definition, 1 volt is an electromotive force with a charge of 1 coulomb. In other words
1 volt = 6.24 X 1018 electrons of electromotive force
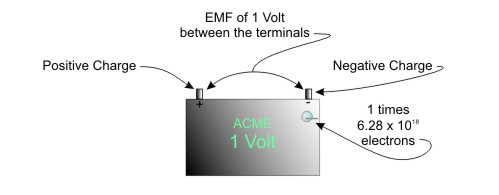
Figure A 1 Volt of EMF means 6.24 X 1018 electrons available to do work
Simply put, this means that 1 volt of EMF means there are 6.24 X 1018 electrons available to do work. The car battery has 12 times this many electrons available. They just sit there until you connect something to the terminals.
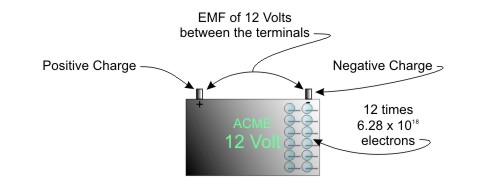
Figure 12 Volts of EMF means 12 times 6.24 X 1018 electrons available to do work
From a practical perspective, we tend not to think about the actual number of electrons in 1 volt of potential and use the volt as the unit of measure.
Electrical Current
Topics covered
- Electron flow
- The unit of measure for electron flow (electric current)—the ampere
Electron Flow
While electromotive force (EMF) is potential difference in charge between two points (for example the battery terminals), electric current is the result of electrons moving in a coordinated manner from one point to another.
In the figure below a few conventions (standard ways of doing things) are followed. A red wire is used for positive and a black wire is used for negative connections.
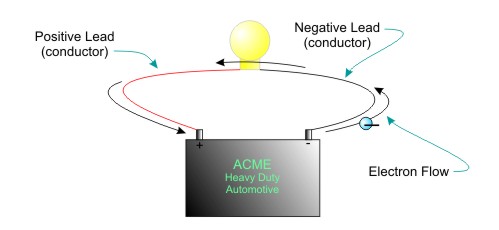
Figure Electric Current is the Flow of Electrons in a Circuit
Unit of Measure for Electric Current—The Ampere
Electric current, or electron flow, is measured in amperes. The ampere, named after André-Marie Ampere (1775-1836) is frequently shortened to amp. By definition
1 ampere = a charge of 1 volt moving past a point in 1 second
We already know that 1 volt is an EMF of 6.24 X 1018 electrons. Therefore, an ampere is 6.24 X 1018 electrons moving past a point in the circuit in 1 second.
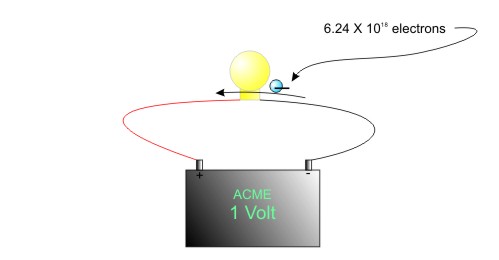
Figure One Amp of Current = One Volt of Charge in One Second
One ampere of current is a very large quantity. Car batteries are capable of producing very large current flows, often as high as several hundred amps.
The next figure represents the quantity of electrons that move when 10 amps of current flows through a circuit connected to a 12 volt battery. Numerically
1 volt at 1 amp = 6.24 X 1018 electrons
12 volts at 1 amp = 12 X 6.24 X 1018 electrons
12 volts at 10 amps = 12 X 10 X 6.24 X 1018 electrons
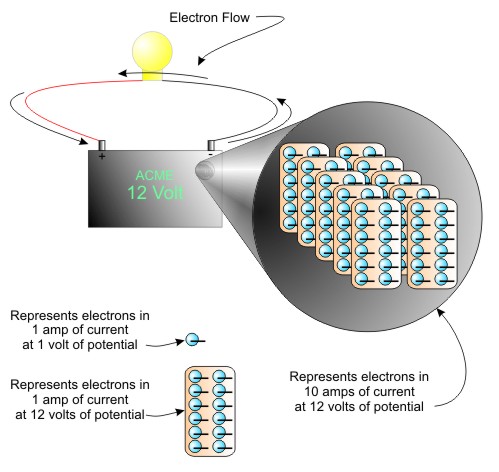
Figure 10 Amps of Current at 12 Volts
That is enough power (you will see this term again in the next activity) to cause serious damage, including melting steel and igniting fires. On the other hand, AA, AAA, C, and D cells only produce a few thousandths of an amp. The same is true for 9 volt batteries.
For More Information
You may want to check these sites for more information on electricity
When you are ready, move to Your Turn