| Intermediate Technology Education | Energy and Power Technology | Pre-Design | Topic 3 | Activity 2 |
Mass
- Describe mass in terms of the amount of matter in an object
- Define weigh in terms of gravitational attraction (a force)
- Distinguish between mass and weight
- Investigate mass in technological problems and solutions
Mass
Mass should not be confused with weight. They are not the same thing. You can be weightless, but you cannot be mass-less.
Mass starts Really, Really, Small!
As you already know, everything is made up of atoms. Atoms are constructed from neutrons, protons, and electrons (and other stuff that we'll ignore).
Atoms are differentiated from each other by the numbers of protons, neutrons and electrons they contain, as in the example below. Each coloured circle represents one atom.
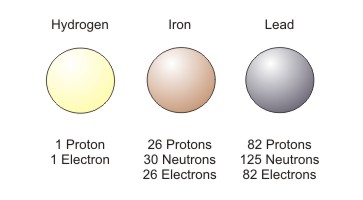
Figure Mass: Each atom has a different mass
The mass of an atom is determined by the number of neutrons and protons that make up the atom. Electrons contribute very little to mass. The more protons and neutrons an atom has, the more mass it has. As the image below shows, iron has more mass than hydrogen, and lead has more mass than iron.
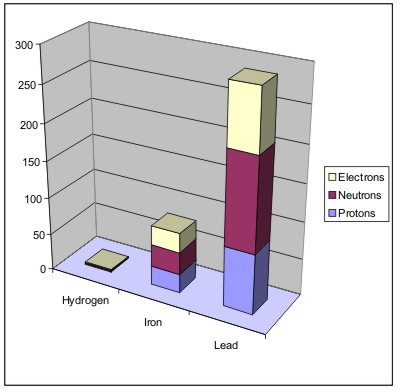
Figure Total Mass of an Atom (total number of protons, neutrons and electrons—electrons add very little to the mass)
Atoms combine to form molecules. While there are only a few naturally occurring atoms, they combine into unlimited numbers of different molecules, which combine to create everything in the universe, including us. The mass of each atom in a molecule contributes to the total mass of the molecule. A water molecule, for example, is made from 2 hydrogen atoms (2 protons, 2 electrons) and one oxygen atom (8 protons, 8 neutrons, 8 electrons) for a total of 10 protons, 8 neutrons and 10 electrons. The total number of molecules in an object times the mass of each atom in the molecule determines the mass of the object. It is the differences in the mass of those atoms and molecules that make some things have more mass than others.
Mass compared to Weight
Suppose you have a cast iron anvil. Iron atoms have a lot of mass, and consequently the anvil has a lot of mass. You are probably thinking that it is also heavy. You would only be correct for some conditions. But how can that be? How are mass and weight related?
Figure Mass
Mass is not dependant on where the anvil is located. Weight, on the other hand, is dependant on location. In the next image, the anvil is shown in three locations. In each location, its mass is the same. but its weight is very different.
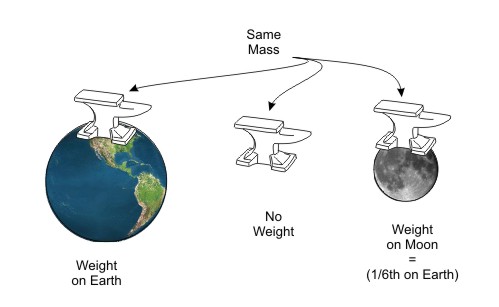
Figure Mass Compared to Weight—Mass is constant, Weight depends on location
Weight depends on gravitational attraction, which is the force that pulls different masses towards each other. We experience this force as weight.
When the anvil is close to the earth, the earth's gravitational force pulls the anvil towards it. If we move the anvil far enough away from earth, the gravitational force is weakened and the anvil has no weight, but it still has the same mass (same number of molecules). If we move it to the moon, it has weight again. Its mass still has not changed, but since the moon has only one sixth the mass that the earth has, its gravitational force is 1/6th earth's and the anvil will have one sixth as much weight on the moon.
Density
Density is how much mass is in a given volume of space. Suppose you have a cubic meter of iron and a cubic meter of lead.
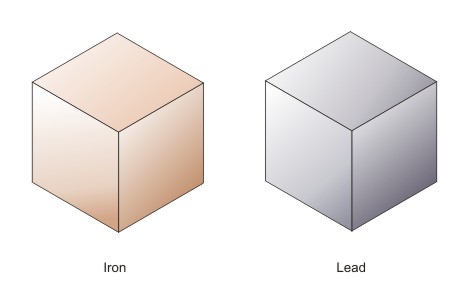
Figure Density [Volume / Mass]
Both have the same volume. From the information above, you know that lead has more mass than iron. The lead cube is denser because it has more mass in the same size block. What about a cubic meter of Styrofoam? Is it more or less dense than the iron?
Unit of Measure for Mass
In the SI system of metric units, which we use, the unit for mass is the kilogram.
How to Measure Mass
A common method for measuring mass is to compare an object of unknown mass to another of known mass. A balance beam is typically used. The principle is illustrated in the next image.
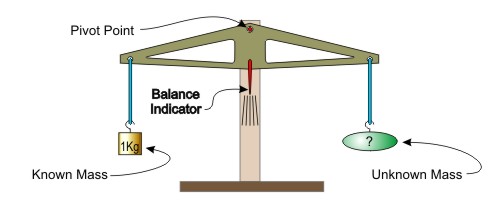
Figure Principle of Balance Beam
As the figure illustrates, by using a balance beam, the mass of an unknown object can be determined by adding known masses to the other side of the beam until the indicator is centered.
A typical science lab balance beam is shown below.
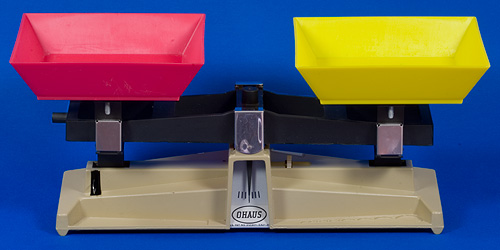
Figure Science Lab Balance Beam
The following images show a selection of known masses.

Figure Selection of Known Masses
The next image show the balance beam (with the trays removed). This makes it easier to see the masses in the photographs
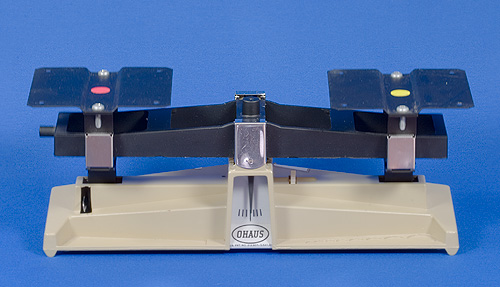
Figure Balance Beam without Trays
The next image shows an unknown mass (piece of aluminium) on the left and a known mass of 20 grams (.02kg) on the right. The pointer is centered so we know the aluminium has a mass of 20 grams.
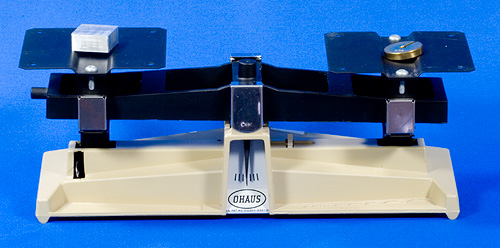
Figure Determine an Unknown Mass
The following video clips show how the mass above was compared to a known mass. Pay attention to the movement of the indicator.
The next activity will look more closely at weight.
For More Information
For more information about the concept of mass, try these sites
- How Force, Power, Torque and Energy Work: What is Mass
When you are ready, go to Your Turn