Before You Start
There are a number of issues associated with recharging batteries.
- The
source must have a higher voltage than the batteries being charged or
current will not flow to the battery. Think of two ponds connected by a
stream. Water will not flow between them unless one is higher.
- With a solar cell, the voltage will drop off as light levels fall. If the
battery has a higher voltage, current will flow in the opposite direction,
discharging the battery.
- Leaving the charging circuit on for too long may result in overcharging
the battery. This could shorten its operational life and for some types,
might cause damage or explosions.
- Another issue is the amount of charging current. Some cells cannot
tolerate high currents and may be damaged. Commercial battery chargers are
specifically designed for the properties of the cells they charge. Never use
a charger which is not designed for the cell!
In this part of the activity, you will only charge the battery for a
short time and you will need to use the number of cells that matches your
solar array.
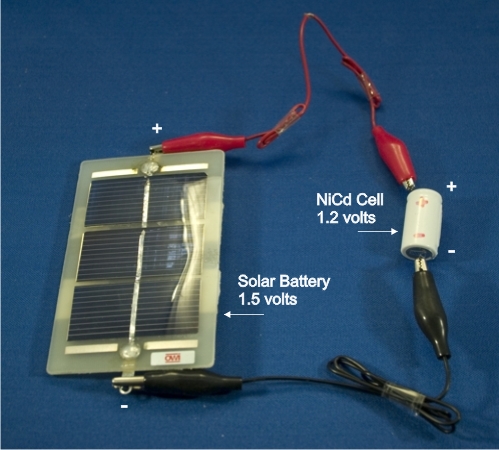
Pictured below are two types of rechargeable cells.
- The first is a battery of Nickel Cadmium cells. These are surplus
wireless phone batteries and each cell has a voltage of 1.2 volts. The
battery can be taken apart and the individual cells used. Either use the
leads on the batteries or tape wire leads to the terminals. Never solder
wires to the cells as there is a risk of explosion!

Figure. Nickel Cadmium cells
- The second is a Nickel Metal Hydride cell in AA format. You can
purchase standard holders and combine the cells in series to make higher
voltage batteries.
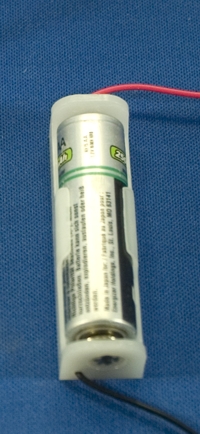
Figure. Nickel Metal Hydride cell
Because of their special charging properties it is NOT recommended that
Lithium Ion cells be used in this activity.